Difference Between Probability Density and Radial Distribution Function
Explain the difference between a plot showing the probability density for an orbital and one showing the radial distribution function. I understand that probability of finding electron in Probability density decreases as distance increases.

Quantum Chemistry Why Are The Radial Wavefunction And Radial Distribution Function Different Chemistry Stack Exchange
Radial Probability Radial Probability Density x Volume of spherical shell 4πr 2 drR 2 nl r Radial probability distribution or Radial probability function.

. The probability distribution function probability function has ambiguous definition. If a given particle is taken to be at the origin O and if ρ N V displaystyle rho NV is the average number density of particles then the local time-averaged density at a distance r displaystyle. Click hereto get an answer to your question - Which of the following statement concerning probability density v2 and radial distribution function 4tr2y2 for a s-orbital of H-like species is correct.
On the other hand probability density psi2 tells us that there is some finite probability that an electron will be found in that very same region of space. Probability density is probability per volume. Probability Density shows that there is a finite non-zero probability of finding electrons at the nucleus.
Saying that the radial distribution function is zero means that electrons will never be found within that volume. It determines the orientation of the orbital in space. It is also known as radial probability density function it is given by 4πr 2 R 2 nl r.
Probability density has a max at the nucleus whereas the radial distribution function is zero at the nucleus and increases to a max then decreases again S orbital shape p orbital shape. The relation between radial probability and radial probability density is given as. Probability distribution functions are defined for the discrete random variables while probability density functions are defined for the continuous random variables.
In the graphs shown in question ψ 2 is shown. Radial distribution shows that there is a zero probability of finding electrons at the. So if we have this over here we have the radial function over our which is the radius the distance rather from the nucleus.
Probability distribution function and probability density function are functions defined over the sample space to assign the relevant probability value to each element. Probability density at a given point means probability per volume in the limit that the volume is infinitesimally small. A v2 is minimum at nucleus but 4tr2y2 is maximum at nucleus.
B v2 is maximum at nucleus but 4tr2v2 is. Radial probability distribution at a given radius is the probability per distance that the event occurs in a infinitesimally thin spherical shell at that radius. Probability density function PDF Cumulative distribution function CDF or probability mass function PMF statement from Wikipedia But what confirm is.
Difference between Probability density and radial distribution. Probability Mass Function PMF. Probability density at a point is defined as probability per unit volume in limit that volume is infinitesimally.
And radial distribution increases to a max and then decreases. Up to 256 cash back Consider the discussion of radial probability functions in A Closer Look in Section 66. In statistical mechanics the radial distribution function g displaystyle g in a system of particles describes how density varies as a function of distance from a reference particle.
Radial probability distribution at a given radius is the probability density of an electron in an infinitesimally thin spherical shell at that radius and is a function of radial distance from the nucleus. A What is the difference between the probability density as a function of r and the radial probability function as a function of rb What is the significance of the term 4 r 2 in the radial probability functions for the s orbitalsc Based on Figures 619. So the plot showing radio distribution the radio distribution function represents a total probability of finding an electron within a didnt spherical shell at a distance are over nuclear.
They may be referred to. Explain the difference between a plot showing the probability density for an orbital and one showing the radial distribution function.

Quantum Chemistry Identifying Radial Probability Distribution Curve Given N And L Value Chemistry Stack Exchange
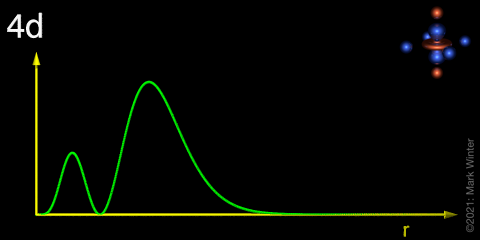
The Orbitron 4d Atomic Orbitals Radial Distribution Function

Radial Distribution Functions For The 2p And 3p Orbitals Of The Al Download Scientific Diagram

Radial Extent Of 4f And 5f Valence Electrons A The Radial Probability Download Scientific Diagram
Radial Probability Density Of The 3s And 3p Orbitals In Atomic Units Download Scientific Diagram

4f And 5d Radial Distribution Functions Of Ce 3 And The Radial Download Scientific Diagram
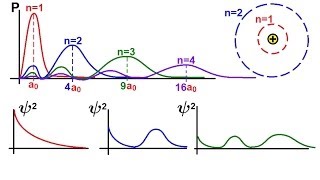
Chemistry Electron Structures In Atoms 26 Of 40 Radial Probability Density Function S Orbital Youtube
Radial Distribution Function Graph Physics Forums
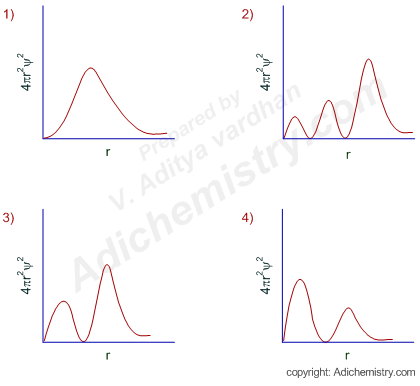
Radial Probability Distribution Curves Atomic Orbitals

Chemistry Electron Structures In Atoms 26 Of 40 Radial Probability Density Function S Orbital Youtube

Computational Chemistry How To Obtain The Radial Probability Distribution Function From A Quantum Chemical Calculation Chemistry Stack Exchange

Li O Li N And Li H Radial Distribution Functions For Li Atom In 80 20 Download Scientific Diagram

1 Radial Distribution Functions For Nd Orbitals Where A0 Is The Bohr Download Scientific Diagram